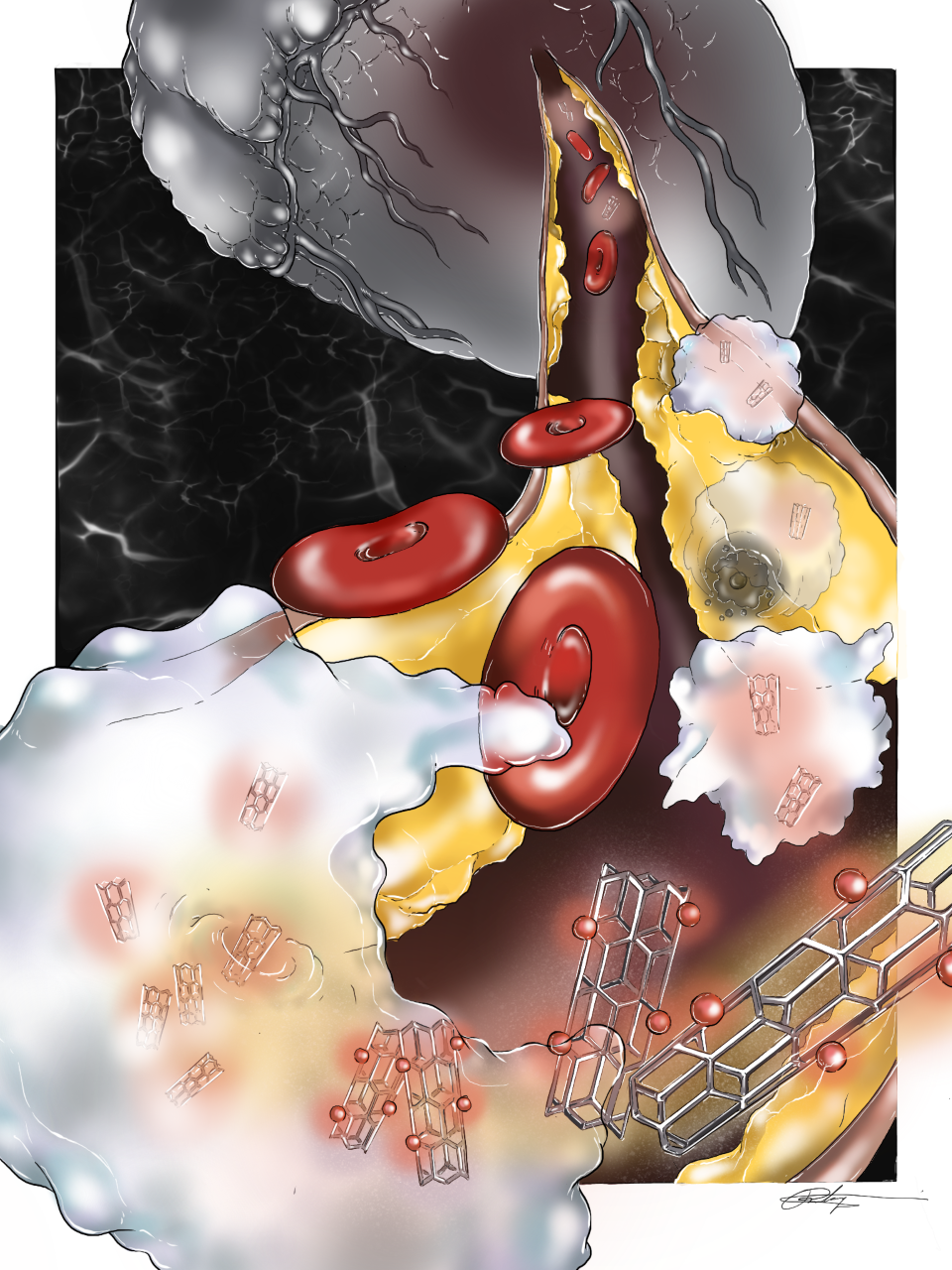
Like a video game ghost chomping along a maze to advance to the next level, a novel nanotech therapy created by scientists at Michigan State University and Stanford University have discovered a way to eat away portions of the plaques that cause heart attacks.
Bryan Smith, associate professor of biomedical engineering at MSU, and collaborating researchers are working on a “Trojan Horse” nanoparticle that can be directed to eat debris, reducing and stabilizing plaque. The research seeks to advance treatment of atherosclerosis, which kills more men and women in America than cancer.
The research results have been published on the cover of the scientific journal, Nature Nanotechnology.
“Fundamentally, our research paper describes a cool story about a ‘Trojan Horse’ nanoparticle that ‘homes’ to the atherosclerotic plaque due to its high selectivity to a particular immune cell type (known as monocytes and macrophages). Once inside the macrophages in those plaques, it delivers a drug agent that reinvigorates ‘efferocytosis’ (a cell engulfing and ‘eating’ cellular debris), thereby removing the diseased/dead cells in the plaque core. By reinvigorating the macrophages, plaque size is reduced and stabilized.”
When clinically tested, Smith said the findings are expected to reduce the risk of most types of heart attacks, with minimal side effects due to the unprecedented selectivity of his nanodrug.
His research is a continuation of studies he worked on while at Stanford University. Those results were previously published in Nature Nanotechnology.
Since coming to MSU, many of Smith’s studies are focused on ways to intercept the signaling of the receptors in the macrophages and send a message via small molecules using nano-immunotherapeutic platforms. Previous studies have acted on the surface of the cells, but the new approach works intracellularly and is showing an advanced level of efficacy.
“We found we could stimulate the macrophages to selectively eat dead and dying cells—these inflammatory cells are precursor cells to atherosclerosis—they are part of the cause of heart attacks,” he explained. “We could deliver a small molecule inside the macrophages to tell them to begin eating again.”
Smith said he believes this approach may help a variety of patients once translated.
“We think this strategy can be further extended to other diseases,” he continued. “The signal to macrophages not to eat diseased cells isn’t unique to only atherosclerosis.
“We were able to marry a groundbreaking finding in atherosclerosis by our collaborators with the state-of-the-art selectivity and delivery capabilities of our advanced nanomaterial platform. We demonstrated the nanomaterials were able to selectively seek out and deliver a message to the very cells needed,” he added. “It gives a particular energy to our future work, which will include using large animal models and human tissue tests to translate these nanomaterials clinically. We believe it is better than previous methods.”
Smith and colleagues have filed a provisional patent for the innovative process.
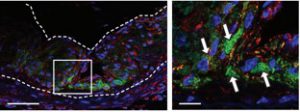
The dotted line outlines the atherosclerotic artery in a mouse; the green represents nanoparticles in the plaque, and red indicates macrophages that the nanoparticles are stimulating to eat the debris.
- Communications contact: Patricia Mrozek, Engineering Communications