Researchers are designing tiny, versatile particles to help doctors better detect and treat the world’s leading cause of death
Originally Published on MSUTODAY on April 10, 2023
MSUToday’s “Ask the Expert” articles provide information and insights from MSU scientists, researchers and scholars about national and global issues, complex research and general-interest subjects based on their areas of academic expertise and study. They may feature historical information, background, research findings or offer tips.
Cardiovascular disease is the world’s leading cause of death, killing nearly 20 million people every year. In the United States, it was responsible for one in every five deaths in 2020, according to the Centers for Disease Control.

It’s no surprise, then, that researchers like Bryan Smith of Michigan State University see a huge opportunity for new, innovative diagnostics and therapeutics to help patients affected by heart disease and other cardiovascular health issues.
Smith is an associate professor in the Department of Biomedical Engineering and director of the MSU T-NIE Lab — the Translational NanoImmunoEngineering Lab — in the Institute for Quantitative Health Science and Engineering, or IQ.
In particular, Smith and his team are developing what are called nanomedicines. These are tiny, versatile particles that researchers are engineering to zero in on problem sites in the body to maximize a treatment’s power and minimize its side effects compared with what’s currently available.
Smith was recently invited by the journal Nature Cardiovascular Research to provide an overview of nanomedicines in the realm of heart health for the research and clinical communities. We’ve caught up with him to ask a few questions about what his work could mean for the general population.
What is cardiovascular disease?

When we talk about cardiovascular disease, it isn’t just one thing. It’s all the issues that can affect the heart and blood vessels as well as the blood. It could be a disorder of the myocardial tissue that’s pumping the blood, or a pacemaker issue, or ischemia, which is a lack of blood supply to a region. There can be problems with blood vessels. Atherosclerosis is the big one, but there are lots of different blood vessel disorders that all fall under cardiovascular diseases.
So it’s a little bit like how we talk about cancer. Cancer isn’t one disease. It’s many different diseases that all have different pathways, but they all fall under one major heading. It’s similar for cardiovascular diseases, except that the differences between them are usually much more obvious.
I recently had a relative with a cardiovascular disease who needed open-heart surgery. That was scary, but it didn’t scare me as much as when I learned another relative had cancer. Hearing that cardiovascular disease is the world’s leading cause of death, I feel like I didn’t fully appreciate its severity. Why do you think that is?
As I understand your question, you’re saying cancer struck fear into you in a way that cardiovascular disease didn’t. I think that’s common, and I think there are a few reasons for that.
One, for a long time — prior to the 1980s, 90s and even into the 2000s — cancer was almost a bad word. Like, we almost wouldn’t say “cancer” and I think that’s because we, as a population, didn’t really understand what cancer was.
Another part of the fear is that cancer is us: Cancer is a malformed, dysregulated version of our cells. Our own cells have gone “bad,” and now they’re attacking us. There’s something intrinsically frightening about that.
“If you’re concerned about cancer, it would be prudent to be at least as concerned about cardiovascular disease — if not more.” – Bryan Smith, associate professor of biomedical engineering
When it comes to cardiovascular disease, on the other hand, I believe people think that it’s basically your plumbing has gone bad. It’s something that just is supposed to happen over time.
It turns out for most cardiovascular diseases, that’s simply not true. The pathogenesis, the pathogenic mechanisms, of a number of cardiovascular diseases actually parallel those of cancer. And that’s why some treatments that are used in cancer can be used in atherosclerosis and vice versa.
Another reason that people aren’t as fearful of cardiovascular disease is because they tend to think that it’s a disease of old age. In recent years, though, we’ve heard about more and more people having heart attacks at 35, 40 years old.
A part of that is we’ve gotten better at detecting these events. But we now know that cardiovascular disease is not just a disease of old age but is something that can happen at any time in our adult lives. I do think it’s fair to say if you’re concerned about cancer, it would be prudent to be at least as concerned about cardiovascular disease — if not more.
Why is cardiovascular disease so prevalent and what makes it so lethal?
Part of this is what we talked about earlier. Cardiovascular disease isn’t one thing. It encompasses a wide variety of disorders and maladies. But also, the cardiovascular system is critical to the function of every tissue in the body for every minute of our lives. And there are so many parts to the cardiovascular system that can go wrong.
There’s the heart, but also the blood vessels and the blood. That’s so much of our body by weight, by volume and by number of cells. So, the diseases are prevalent because the cardiovascular system makes up so much of who we are.
The reason that cardiovascular diseases are so deadly is because every cell, every cubic millimeter of our body depends on our heart to pump and perfuse blood and nutrients that we need to live. Thinking in that way, then, it becomes kind of obvious that if something goes wrong, it could be lethal, especially if a cardiovascular-related problem occurs in the brain, the heart or another vital organ.

What are the opportunities for nanomedicine in the fight against cardiovascular disease?
I believe that there are tremendous opportunities for nanomedicine to impact cardiovascular diseases. I really think we’re just starting to scratch the surface.
To back that up, last year alone, more articles were published in the field of cancer nanomedicine than have ever been published in the field of cardiovascular nanomedicine. To me, that spells opportunity.
One of the key limitations in current diagnostics and therapeutics is their propensity to cause unintended consequences or adverse side effects. That’s one of the biggest issues in medicine. There are many medications that could be absolutely outstanding for treating a disease, but they’ve been dropped because they have terrible side effects.
In pretty much every medicine ad you see on TV, they’ll show something like people playing with their kids while they talk about all the horrible side effects that medication may cause. By law, they have to tell you what the side effects are and it’s often dozens of different things, sometimes even including consequences like blindness and death. Afterward you’re left thinking, “Would I really want to take that medication?”
What if you could avoid most side effects by getting the medication exactly where you want it to go and nowhere else? That’s what’s so exciting about nanomedicine.
Nanomedicines can be engineered to be extremely, exquisitely selective for particular cells or particular organs, which would then lead to reduced or ideally no side effects. And this is where nanomedicine started. The first nanomedicines didn’t actually treat the disease any better than the regular medicine, but they reduced side effects.
Another area that’s really exciting is nanomedicines can be multifunctional. A nanomaterial can contain a drug, or even lots of drug molecules, but it also can have certain mechanical, immunological and even electrical properties. That’s really attractive, especially in cardiovascular tissues where mechanical, electrical and immunological properties are all critical to proper function.
Can you share an example of what you’re working on in cardiovascular nanomedicine?
Absolutely. One of the things we’re doing is stimulating immune cells essentially to clean up the mess within atherosclerotic plaques.
Atherosclerosis is really a critical area of cardiovascular disease. When most people think about cardiovascular disease, they’re thinking about heart attacks. Heart attacks are the primary cause of mortality in cardiovascular disease and atherosclerosis is what causes the vast majority of heart attacks.
That makes atherosclerosis one of the key areas to treat if you want to reverse this statistic of cardiovascular disease being the number one killer of humans worldwide. That’s what we’re doing, and we colloquially refer to it as “taking out the garbage.”
How does that work?
The idea is that cells die throughout your body and typically immune cells called macrophages clean up these dead and dying cells. There’s a constant, healthy process called phagocytosis, in which macrophages eat the dead cells.
That doesn’t happen in an atherosclerotic plaque. There’s a buildup of dead cells, and the macrophages get lazy and don’t do their job. When you need these macrophages the most, they’re not working properly.
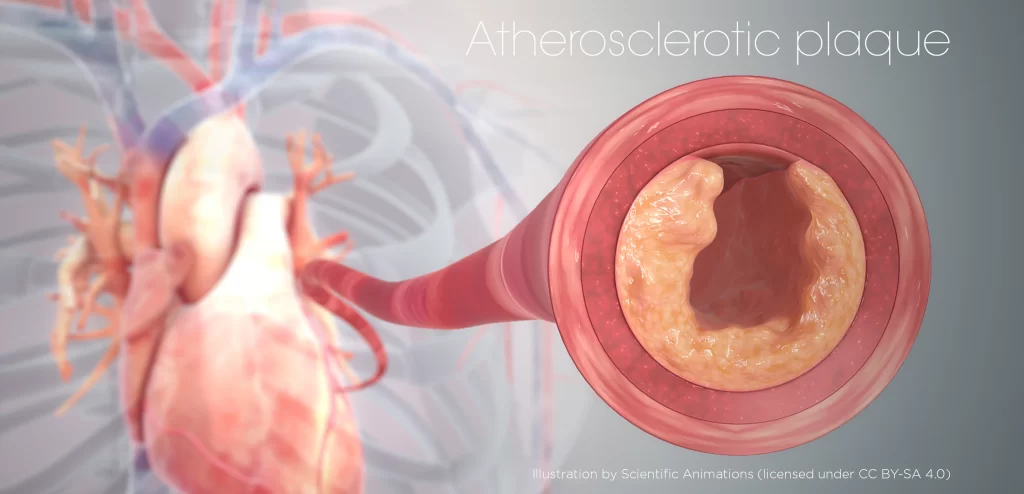
Instead, there’s a buildup of dead cells that, along with diet and other factors, drives a chronic inflammatory response. The plaque grows bigger and bigger, especially the soft center, making it more and more dangerous because it gets closer to rupturing, which would cause a heart attack.
What we’re developing is a nanomedicine that specifically and selectively targets macrophages and monocytes (the precursors to macrophages), but not other cells. It delivers a drug to those cells that stimulates them to again become good garbage collectors and clean up the plaque.
We’ve shown in preclinical trials that this nanotherapy decreases plaque size, stabilizes plaques and lowers inflammation in the disease site. That’s likely to be critical in the continuing treatment and care of plaques, assuming that, after treatment, patients eat appropriately, exercise and do all the other things necessary to help reverse atherosclerosis.
What that decreased inflammation actually means in the long run is a question we have not yet explored and we hope to examine with animal models and, ultimately, with patients.
Where are nanomedicine therapies for cardiovascular disease in the pipeline now? How long before they might be ready to treat patients?
We’re still early in this field, especially compared with cancer nanomedicine. Looking at U.S. and European clinical trials combined, cancer nanoparticles had around 350 clinical trials, whereas cardiovascular nanoparticles had about 25. The difference is stark.

But cardiovascular trials do exist, and some are really interesting. There was one using gold-silica nanoparticles with what’s called photothermal therapy in a relatively small patient cohort for atherosclerosis.
With this small cohort, the trial found that these particles did a better job than stents, which is exciting because stents are a major part of cardiovascular health care. Now, that trial has to be repeated on a much larger number of patients to be validated. But that’s just one small example and, as I mentioned, there are 25 nanomaterial-related clinical trials out there right now.
I think we’ll see quite a few more clinical trials within the next five to 10 years, and I hope individuals will be on the lookout for those and consider enrolling if it’s something that they and their physicians agree could help.
Is there anything else you want people to know about nanomedicines and cardiovascular disease?
If this interests someone, they can get involved in local foundation chapters that are dedicated to cardiovascular health. Despite being the world’s number one cause of death, cardiovascular disease doesn’t receive nearly the same level of support that cancer does.
I think heart disease deserves that support and if you believe it, too, support your local chapter of the American Heart Association. Write to your government representatives to make an argument for increasing funding for the National Heart, Lung and Blood Institute.
People also can check out my website and the websites of other researchers doing work in this area and send us questions.
By: Matt Davenport