MSU researchers awarded a $750,000 grant by JDRF to develop a new immune imaging method that could bolster promising therapies
Originally published on MSUTODAY on Nov 27, 2023
Researchers at Michigan State University have teamed up to create a new way to image immune cells in living subjects that could enable new treatment opportunities and better outcomes for patients with Type 1 diabetes.
In particular, the new approach could help bolster the success of cell transplantation strategies used to treat and cure Type 1 diabetes, as well as broaden the pool of patients who are eligible for this promising clinical procedure.
“When transplants work, they work great,” said Bryan Smith, an associate professor in the Department of Biomedical Engineering. “But they can be rejected by a patient’s own immune system, which is obviously a major problem. Things can spiral quickly.”
Smith also works in the Institute for Quantitative Health Science and Engineering, or IQ, where his lab is developing new approaches to diagnose and treat disease, specifically cancer and heart disease.
The researchers specialize in creating tiny, engineered particles that team up with immune cells to help spot or remedy problem sites inside the body.
Although Smith had never applied his approaches to diabetes before, the methods represented the kind of innovative ideas an organization called JDRF was looking to support.
Established in 1970 by parents determined to find a cure for their children with Type 1 diabetes, JDRF is the leading global organization funding Type 1 diabetes research, working to “advance life-changing breakthroughs to prevent, treat and cure Type 1 diabetes and its complications.”
“I am not certain how they identified me, but they reached out and said our work might be a good fit,” Smith said. JDRF let him know about a grant opportunity and invited him to chat if he had any questions about it.
Fortunately, he didn’t have to look far to find those collaborators right at MSU to create a proposal that earned the team a $750,000 grant from JDRF.
Joining the effort are Anna Moore, director of the Precision Health Program and assistant dean in the College of Human Medicine, along with Peter Wang, an assistant professor in the Precision Health Program and the Department of Radiology.
Before joining MSU, Moore and Wang worked together at Massachusetts General Hospital and Harvard Medical School developing imaging techniques for diabetes.
Karl Olson, an associate professor and chair of the Department of Physiology, is another collaborator, whose research focuses on the type of pancreatic cells used in transplants for Type 1 diabetes.
The team also includes Cheryl Rockwell, an associate professor in the Department of Pharmacology and Toxicology, who is the acting director of the Applied Immunology Center for Education and Research, or AICER, at MSU.
Rockwell’s laboratory uses a variety of tools and techniques that will help the team understand how its new approach is affecting immune cells.
“In a lot of ways, Michigan State is a unique research environment,” Rockwell said. “It’s exceptionally collegial and collaborative. We strengthen one another.”
The Problem
Type 1 diabetes is an autoimmune disease. A person’s own immune system attacks the cells in the pancreas that produce insulin, which regulates blood glucose or blood sugar. As a result, people with Type 1 diabetes lose their ability to regulate their blood sugar levels. The condition typically requires constant management through insulin injections and monitoring of food intake and physical activity.

The majority of patients are diagnosed before adulthood, which is why the condition was also once called juvenile diabetes (JDRF was previously known as the Juvenile Diabetes Research Foundation). But we now know a diagnosis can also come at a later age.
More than 1 million people in the U.S. have Type 1 diabetes and, currently, there is no cure.
But promising treatments called beta cell or islet transplantations are being developed. In these procedures, doctors take islets of healthy pancreatic tissue that contain insulin-producing cells — called beta cells — and transplant them into patients with Type 1 diabetes.
The islets typically come from a donor pancreas, but emerging research is also using stem cells to generate insulin-producing cells for transplants.
“Results from ongoing clinical trials look very promising,” Giraldo said.
Regardless of where the cells come from, however, there’s a chance the recipient’s immune system might see a cell transplant as a threat and attack it.
This is also a concern with more conventional whole organ transplants, and scientists have developed what are known as immunosuppressive drugs to reduce the risk of transplant rejections.

These drugs, however, also compromise a patient’s ability to fight off other threats, such as infection or cancer cells.
This risk, combined with a current scarcity of donor islets, has meant that transplants are limited to the most severe cases of Type 1 diabetes. In these cases, extremely low blood sugar levels can lead to seizures, loss of consciousness, coma and death.
With better transplant screening methods, medical researchers could build trust and confidence in transplant therapies. That would, in turn, help make these potentially revolutionary procedures more accessible — and, ideally, available to all Type 1 diabetes patients, the researchers said.
“Right now, it’s only the highest-risk patients who are getting transplants,” Smith said. “What we’re doing could lead to a much lower bar for needing a transplant and help us get transplants into patients earlier in their disease progression.”
Furthermore, transplants can still be rejected over time, even when immunosuppressive drugs are being used. Currently, it may not become apparent for weeks or months after a procedure whether a transplant is being rejected. By that time, the transplanted cells would be lost.
“If we could know early on — within a few days — whether or not a transplant will be rejected, we would lower the risk associated with the procedure,” Smith said. “We could better guide the course of treatment to avoid transplant failure.”
Immune monitoring and imaging technologies such as those developed by Smith and his team could allow researchers to determine if immunosuppression is failing and intervention is needed to protect the transplants before they fail.
The proposal
One of the challenges in determining how successful a transplant will be is that there are a variety of different immune cells involved in rejection. Researchers want to know what each cell type is doing and how they work with or against each other during different stages of a transplant rejection.

“It’s a daunting task,” Moore said. “It’s part of the reason why transplants aren’t considered a cure at this point.”
While at Harvard Medical School and Mass General, Moore and Wang developed ways to image viable islets both before and after transplantation. The team’s new project, led by Smith, will look at another part of the puzzle: Namely, how do immune cells in a recipient respond to the transplanted islets and to immune interventions delivered to control that response?
But there’s diversity among those immune cells that also presents challenges.
“It’s becoming more and more clear that there are multiple important cell types involved in rejection,” Smith said. “So, while we might want to look at multiple cells in the clinic, it can be very difficult to bring patients in for multiple scans.”
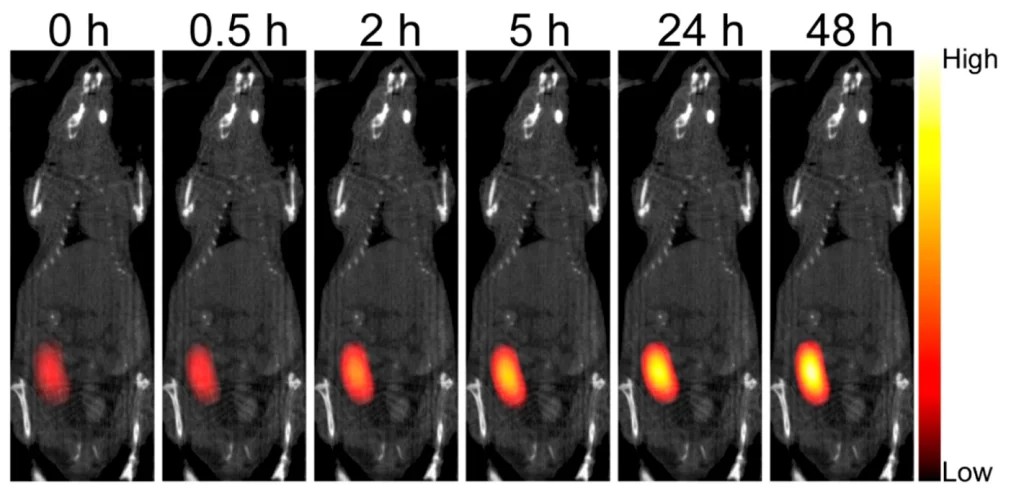
The team is turning to an emerging imaging technique called magnetic particle imaging, or MPI, to study multiple cell types at once. This technique was first introduced less than two decades ago, but it’s yet to be applied to screening beta cell transplants, Smith said.

MPI uses tiny magnetic particles, and the team’s plan is to add these nanoparticles, made from iron oxide, to immune cells. If you think about how big a raisin is in comparison to a person, that’s roughly the size of an iron oxide nanoparticle compared to a cell.
Next, the team will inject these nanoparticle-carrying cells into mice with transplanted islets, providing a model to study immune response to transplantation.
These nanoparticles respond to magnetic fields, which the team will take advantage of to track, visualize and study the cells that are carrying them using MPI. To keep tabs on multiple cell types at once, the team will load different immune cells with different types of iron oxide nanoparticles. Smith’s team also is developing software that will allow them to distinguish between different combinations of cells and nanoparticles.
“We’re starting with two cell types but, ultimately, we want to do more,” Smith said. “Right now, we’re focused on demonstrating these imaging capabilities that nobody’s shown before to develop a platform that can see which different cell types are trafficking to transplant sites.”
The potential
Attempting to do something that’s never been done before comes with both obstacles and opportunities.
For instance, because the team is starting from square one, they must prove that the iron oxide particles — which are generally recognized as safe — don’t actually harm immune cells.

“We don’t think they will, but you still need to do the experiment,” said Rockwell. If these technologies are to be used for monitoring human patients, this is a crucial step to ensure they do not adversely affect immune systems.
“No one’s done this before and, whenever you’re in that situation, you need to consider all the possibilities,” Rockwell said.
For the team, though, that doesn’t mean just thinking about the possible pitfalls along the way. It also means thinking about beneficial possibilities, such as different applications of this new technique in the future.
Both Smith and Rockwell see potential for their MPI approach in certain inflammatory diseases, including some forms of cancer and heart disease. Moore also talked about how it could become a tool to help realize the broader goals of precision health.
“We hear a lot about precision medicine: using the right drug at the right time to treat the right patient. But when you talk about treatment, the person is already sick and we’re reacting,” Moore said. “What we’d like to do is shift the focus to early detection, prediction and prevention, to form the basis for precision health.”
In fact, this technology could also be used to detect the progression of the autoimmune response seen in Type 1 diabetes before the onset of disease, Giraldo said. JDRF hopes MPI can be leveraged in the development of disease-modifying therapies which aim to delay, stop or reverse the progression and onset of the disease.
“One such therapy known as TZIELD (teplizumab-mzwv), developed with support from JDRF and recently approved by the FDA, was shown to delay the onset of Type 1 diabetes in those at risk by two or more years,” Giraldo said.
And Smith sees more promise for the team’s MPI approach in Type 1 diabetes beyond offering doctors a new tool for screening transplants.
Because islet transplants are such an active area of research, many different transplant approaches are in development.
The imaging technology could help accelerate that development, especially for techniques that avoid the use of broad immunosuppression by enabling rapid, standardized screening of transplants for immune rejection in animal and human trials. That would, in turn, expand the population of people with Type 1 diabetes that can receive a beta cell transplant and potentially deliver a cure for Type 1 diabetes.
The researchers expect that this new technique won’t just provide a new way to visualize immune cells, but it could provide a brand-new outlook for diabetes research.
Also discussed on WILX