In a new paper published in EMBO Reports this month, an international team of scientists, including Dr. Aitor Aguirre at MSU’s Institute for Quantitative Health Science and Engineering, has identified a novel signaling pathway critical for aging in the heart. Therapeutic targeting of this pathway was sufficient to slow down the aging process in human heart cells and mice.
One of Dr. Aitor Aguirre’s main research lines at IQ is to develop stem cell-based models to create in vitro organs and cells for modeling human disorders, including heart aging.
“We are interested in the cardiovascular system and the heart because cardiovascular disease is the number one killer in the developed world, including the US,” says Dr. Aguirre.
“Engineering these complex cellular systems using human stem cells allows us to investigate aspects of disease that are not approachable by conventional means,” he adds.
As we get older, our hearts become prone to not working correctly due to decreased renewal and death of heart cells, leading to decreased flexibility and adaptability of the organ as we age. In order to repair the “gaps” left by dead cells, our bodies fill them with scar tissue and fibroblasts, leading to a gradual loss of function which might eventually cause cardiovascular disorders. This loss of function is displayed in exercise – in the heart, the maximum beats per minute decrease as a function of age. For example, a 20-year-old’s heart can output four times its resting capacity, whereas an 80-year-old can output only two times their resting capacity. Furthermore, this age-related loss of function might make us more prone to cardiovascular disease with age (40 percent of deaths between the ages of 65 and 74 are due to heart disease, making it the main cause of death in developed countries).
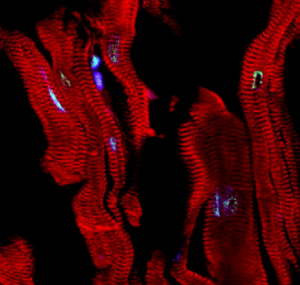
Immunofluorescence micrograph of aged human heart tissue. Cardiomyocytes (red) start to exhibit signs of DNA damage (green) due to accumulation of the bioactive lipid sphinganine.
In the paper, titled “Loss of genomic integrity induced by lysosphingolipid imbalance drives ageing in the heart,” an innovative new model of accelerated vertebrate aging was used – the Killifish. These animals have the shortest lifespan across all known vertebrates, about 3 months, and recapitulate many hallmarks of aging observed in humans. Using untargeted mass spectrometry and computational methods, the authors discovered several metabolites present in old Killifish that were not present in their young counterparts. Among these metabolites was the bioactive lipid sphinganine, a toxic byproduct of sphingolipid metabolism that increased dramatically in the heart and serum of aged fish. Further studies in human stem cell-derived cardiomyocytes revealed that sphinganine was a major driver of cardiac aging and cardiomyocyte death by promoting genomic instability and senescence through aberrant increased histone acetylation. Drugs such as curcumin (a natural product present in turmeric spice and popular in traditional Asian medicine) inhibit histone acetyltransferase activity, and were sufficient to dramatically slow down the damage produced by sphinganine in the heart, and thus slow down the heart aging process.
This research opens the door for novel innovative therapies that could potentially extend human lifespan and improve quality of life for the aging population.
Other authors included: Gaurav Ahuja, Deniz Bartsch, Wenjie Yao, Simon Geissen, Stefan Frank, Nicole Russ, Jan-Erik Messling, Joanna Dodzian, Natalia Emilse Vargas, Anna Klinke, Aleksandra Trifunovic, Susanne Brodesser, Argyris Papantonis, Agapios Sachinidis, Stephan Baldus, Juergen Hescheler and Leo Kurian at the University of Cologne; Joscha Sergej Muck and Dario Riccardo Valenzano from the Max Planck Institute for Biology of Ageing; Christoph Dieterich at University Hospital Heidelberg; Michael Petrascheck at the Scripps Institute; Mohit Jain and Kim Lehmann at the University of California, San Diego.
This work was funded by the NRW Stem Cell Network, the Cologne Cluster of Excellence, German Heart Association, Else Kröner-Fresenius-Stiftung, Deutsche Forschungsgemeinschaft University of Cologne, Koeln Fortune funding, Grants4Targets initiative by Bayer, the National Institutes of Health (NIH/NHLBI) and the Max Planck Institute for the Biology of Ageing.


