Sangbum Park
Contact
spark@msu.edu
Office: 517-353-2479
Lab: 517-353-2490
IQ DIVISION – Developmental and Stem Cell Biology
Park Lab
Departmental AFFILIATIONS
About
Sangbum Park is an Assistant Professor in the Department of Medicine and in the Department of Pharmacology & Toxicology. His lab’s research focuses on understanding fundamental principles of adult tissue regeneration during homeostasis and repair by using in vivo imaging.
He received B.S. in Animal Biotechnology and Ph.D. in Stem Cell Biology at the Seoul National University in South Korea. Dr. Park completed his postdoctoral training with Dr. Valentina Greco in the Department of Genetics at Yale University. As a postdoctoral fellow, Dr. Park established a live cell imaging approach to study tissue repair in live mice and applied this innovative technology to determine how distinct cells of different fates coordinate their behaviors to promote wound repair.
The Park Lab
Regeneration is essential for multicellular life. Many of our adult tissues, such as skin, blood and intestine, undergo constant regeneration to support homeostasis as well as repair injuries. Regeneration is a complex process that requires the participation of multiple cell types, including tissue-resident stem cells. Impaired stem cell function is known to severely impact tissue recovery. However, it remains unclear 1) how other coexisting cells in the tissue, known as the niche, coordinate with stem cells during the repair process and 2) whether interactions between stem and niche cells cause functional behavioral changes in the stem cell population. The challenge in addressing these questions has been an inability to follow the behaviors of different cell types at the same time in vivo and identify functional interactions between stem cells and the niche that regulate stem cell behaviors. Skin is an ideal system to study cellular behaviors of tissue repair because of its accessibility and well-characterized stem cell and niche populations.
In the Park lab, our long-term goal is to discover fundamental principles that underlie skin regeneration in a live animal. To achieve this goal, we utilize in vivo imaging with multiphoton laser microscopy and genetic mouse models to visualize and manipulate specific cell types within the skin of live mice.
Ongoing Research Projects
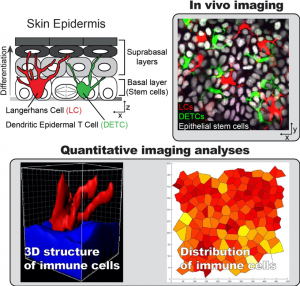
How immune-epithelial interactions maintain organ integrity?
To understand how homeostasis is maintained in an organ composed of multiple cell types, we investigate the key cell populations that exist in close proximity to the epithelial cell pool. In the epidermis, epithelial stem cells are closely intermingled with two resident immune cell populations: dendritic epidermal T cells (DETCs) and Langerhans cells (LCs). These immune populations are known sentinels for pathogen infection, however the extents to which they regulate, or are regulated by, their epithelial neighbors have not been examined. To answer this question, we engineered a fluorescently tagged mouse model that uniquely marks immune and epithelial cell populations (TriColor mice). By using this mouse line, we can coordinately track both immune cell populations and epithelial cells within the same tissue. These approaches will resolve whether distinct tissue types that are interspersed in the epithelium serve as regional check-points to locally control the efficiency of regeneration during homeostasis, thus revealing additional layers of control, and improving our understanding of regeneration at the level of the organ.
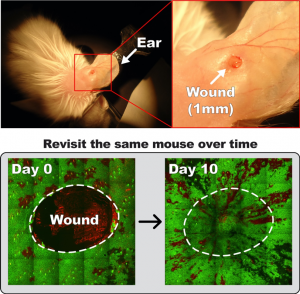
How distinct tissue types coordinately rebuild damaged tissue de novo?
The wound repair involves diverse actions, including making tissue de novo as well as inflammation. It is still elusive whether and how reorganization of the tissue-residence immune cells is coordinated with repair-induced epithelial cell behaviors and how immune-epithelial interactions contribute to wound repair. To address these questions, we utilized previously established in vivo wound imaging (Park et al. Nature Cell Biology 2017) with the Tricolor mice to capture dynamics of both immune and epithelial cells simultaneously during wound repair. This approach will reveal the relationship between the spatiotemporal changes of immune cell organization and the behavioral changes of epidermal cells during wound repair in a live mammal. Furthermore, our studies will elucidate the roles of resident immune cell populations, and their interactions with epithelial cells, during the initiation and progression of skin wound repair.
Our research is highly innovative because of our unique ability to study mammalian skin cells in a live unperturbed animal allow us to answer questions in biology that have been out of reach due to technical limitations. The research we are proposing will not only provide unprecedented insight into the cell biology and molecular mechanisms of tissue regeneration, but will also shed light on disease pathology and potential treatment strategies.